Zika paper-based rapid diagnostic test
Dr. Keith Pardee
A paper-based diagnostic device to detect viral threats
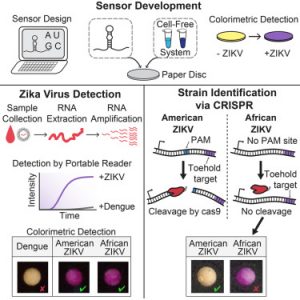
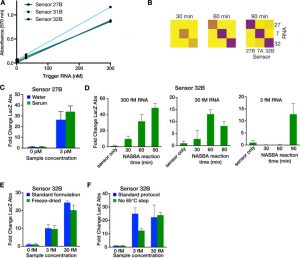
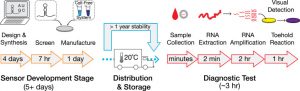
A Zika paper-based diagnostic device is a molecular diagnostic tool embedded into paper that uses blood, urine or saliva to provide results in a short time. It is composed of three components: An amplifier for the genetic sequences found in the patient sample; a toehold switch sensor to recognize if the sequences found are from Zika virus, and a third component to determine the strain of the virus. This product is still a proof of concept, and additional testing is needed to ensure safety and efficacy before actual deployment.
Regions vulnerable to this pandemic threat (South America, Central America and the Caribbean – Countries: Brazil, Colombia, Puerto Rico)
Unknown
ZIKV Detect™ 2.0 IgM Capture ELISA is the only product validated by the FDA for Zika diagnosis.
Goal 3: Good health and well-being
Trained healthcare professionals in need of rapid diagnostic tools