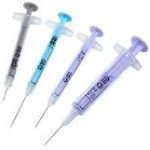
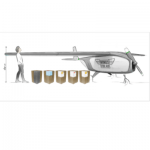
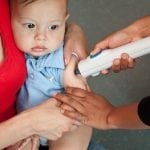
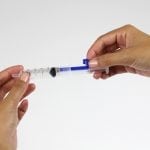
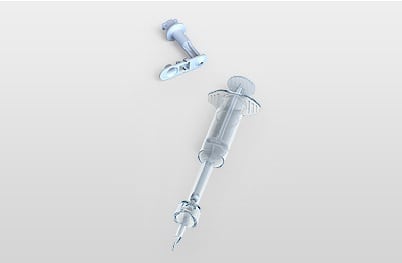
Star ID device
Star Syringe
The Star ID device enables consistent accurate Intra Dermal (ID) injection while at the same time facilitating access to all vial sizes and ampules without any additional devices or manipulation.
This product is discontinued.
Star Syringe has a global partnership with a group of manufacturers in target regions. Specifically: Bangladesh, China, India, Indonesia, Nigeria, Pakistan, Saudi Arabia, Turkey, United States, Vietnam
Unknown
Varies with respect to licensee, quantity and location.
PATH Uniject
Increased vaccination reach through dose sparing of vaccines with limited supply. Decrease potential of disease transmission associated with sharps injury.
Healthcare workers delivering vaccinations and therapeutics in low resource settings.